vaccine delivery system pdf
The recent success of mRNA vaccines in SARS-CoV-2 clinical trials is in part due to the development of. At least six projects produce a DNA vaccine using the S protein gene against COVID-19 at various stages of the clinical trial to evaluate the safety.
Store the vaccine appropriately 2.
. A short summary of this paper. Contact the Department of Health Tip. An overview of mRNA delivery systems and the lipid nanoparticle delivery systems used in the current SARS-CoV-2 vaccine clinical trials are presented and an analysis of the determinants of the performance of lipid nanoparticles in mRNA vaccines is analyzed.
To select several vaccines hold down Ctrl on your keyboard. This has the potential to have multiple benefits to both public health and livestock animal welfare. Vaccine replenish the container with dry ice pellets sized 10 mm to 16 mm within 24.
One objective of the WRAC Fish Immunology Project was to test novel delivery systems for fish vaccines. In addition to antigenic stimuli liposomes can also be designed to. Disadvantages of these vaccines are the need for an efficient delivery system low immune responses compared with live vaccines and the possibility of toxicity due to repeated injection doses.
Before administering vaccines all personnel who administer vaccines should. New staff orientation Annual education requirements Ongoing education. However with little information available on how the system design approach has been used making a case for investment can be challenging.
FOCUS ON INFLUENZA Bruce G. The recent success of mRNA vaccines in SARS-CoV-2 clinical trials is in part due to the development of lipid nanoparticle delivery systems that not only efficiently express the mRNA-encoded immunogen after intramuscular injection but also play roles as adjuvants and in vaccine reactogenicity. Enzymatic barriers for GI peptide and protein delivery system.
Carrier systems such as liposomes microspheres nanoparticles dendrimers micellar systems ISCOMs plant-derived viruses which are now being investigated and developed as vaccine delivery systems are reviewed. Be used within 1 month 31 days. Safety of vaccine administration.
Most vaccines require co-delivery of an adjuvant in order to generate the desired immune responses. 4 MAY 20 2002. Thawed vaccine cannot be refrozen.
If using the thermal shipping container to store. RESEARCH ON NEW VACCINE DELIVERY METHODS. Delivery of antigens from oil-based adjuvants such as Freunds adjuvant lead to a reduction in the number of doses of vaccine to be administered but due to toxicity concerns like inductions of granulomas at the injection site such adjuvants are not widely usedFDA approved adjuvants for human uses are aluminium hydroxide and aluminium.
Thus new adjuvants or self-adjuvanting vaccine delivery systems are required. In particular they describe novel polymeric microneedle systems for delivery of DNA vaccines and review nasal and oral delivery systems for vaccines paying particular attention to delivery system design packaging and ease of administration. FOCUS ON INFLUENZA 1o 7f 7.
For example during H1N1 once vaccines became widely available pharmacies played an important role in. Healthcare system and successful delivery of this vaccine will need to incorporate new types of sites and approaches for vaccine delivery. The strategy for transforming vaccine delivery systems is aimed at the following critical success factors for immunization services.
Multiple delivery systems are being explored both developmental and clinical phases and can open the door for practical development of nasal vaccine delivery systems. Vaccine suspension of weakened killed or fragmented microorganisms or toxins or other biological preparation such as those consisting of antibodies lymphocytes or messenger RNA mRNA that is administered primarily to prevent disease. Vaccines evoke immune responses in patients leading to the production of antibodies and protection against future disease.
The project is focused on developing a new veterinary vaccine delivery system based on the development of an attenuated T. Vaccine strategies have been effective at stimulating specific immunity in the laboratory when injected by IP or IM methods more work is needed to develop better delivery systems and to overcome potential regulatory concerns. Full PDF Package Download Full PDF Package.
Crit Rev Ther Drug Carrier Syst 116195. For Frozen vaccine contact Merck at 1-800-637-2579. Liposomes can be used as carriers for vaccine delivery where antigenic stimuli are 1 encapsulated in the core 2 embedded in the bilayer or 3 adsorbed or engrafted to the outer surface.
These factors can be significantly influenced by the application of new technologies. Between 2C and 8C 36F and 46F the vaccine must. 37 Full PDFs related to this paper.
A system design approach offers a framework for analysing designing and implementing solutions to improve the performance of a vaccine supply chain system. Simplicity and efficiency of vaccine delivery. 466 BIOTECHNOLOGY AND BIOENGINEERING VOL.
This re-view will focus on promising approaches to augment the efficacy of DNA vaccines namely new delivery systems and alphavirus replicons and possible synergy between the two. Receive competency-based training Have knowledge and skills validated Integrate competency -based training into. We present an overview of mRNA delivery systems and.
194 Vaccine delivery systems based. The intranasal vaccine delivery system induces both mucosal and systemic immune response which avoids entry of pathogen in all mucosal routes. A vaccine can confer active immunity against a specific harmful agent by stimulating the immune system to attack the agent.
This evidence brief provides government decision-. Dispose of the dry ice according to the manufacturers directions. These new technologies enable the formulation of vaccine particles containing vaccine antigens without loss of their biological.
Gondiistrain that can be customized to express vaccine antigens of other pathogens. Weniger MD MPH Associate Editor Vaccine Second WHO Consultation on Gl obal Action Plan for Influenza Vaccines GAP-II 12-14 July 2011 Geneva Switzerland Page 1 of 15 Slides 1-77 plus 12 backups RESEARCH ON NEW VACCINE DELIVERY METHODS. The report searches all vaccines unless specified.
DNA Vaccines DNA Vaccine. Vaccines are needed to play a key role in addressing emerging and re-emerging pathogens Development and registration of commercial vaccines is long 10-15 years and costly 2B Vaccine technology has changed little in 200 years Innovative technologies have the potential to fundamentally change the delivery of vaccines for both. Contact the distributor 3.
Up to 8 cash back Recent years have seen the development of novel technologies that use nanoparticles and microparticles to deliver vaccines by the oral and microneedle-based transdermal route of administration. PDF 230K Actions. However many currently available adjuvants are non-biodegradable have limited efficacy andor poor safety profile.
Have many uses including drug delivery for cancer and vaccination with antigens or DNA. The system returns results faster when you use more specific search criteria. Equity in access to new vaccines.
Vaccines are the preparations given to patients to evoke immune responses leading to the production of antibodies humoral or cell-mediated. Ent that the potency of DNA vaccines needs to be increased if this technology is to be effective in large animals including humans. An overview of the immune system with respect to induction of mucosal immune responses is provided and a number of microbial and synthetic strategies that have been investigated as adjuvants or delivery systems for mucosal vaccine development are provided with a focus on the delivery of vaccines via the oral route.
When vaccine administration recommendations are updated. The development of vaccine adjuvants and the emerging area of.
Systems Approach For Better Education Results Saber
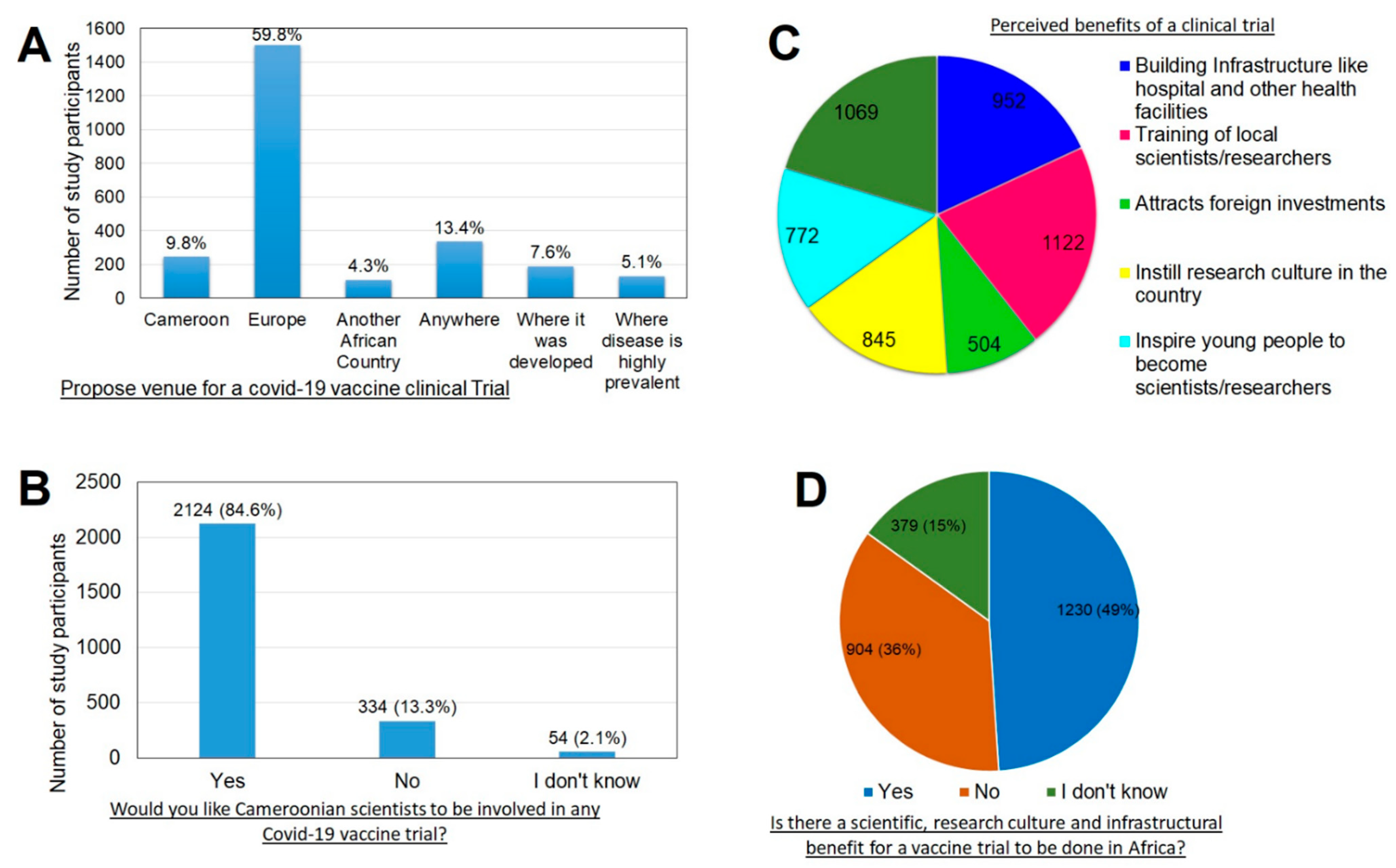
Vaccines Free Full Text Assessment Of Vaccine Hesitancy To A Covid 19 Vaccine In Cameroonian Adults And Its Global Implication Html
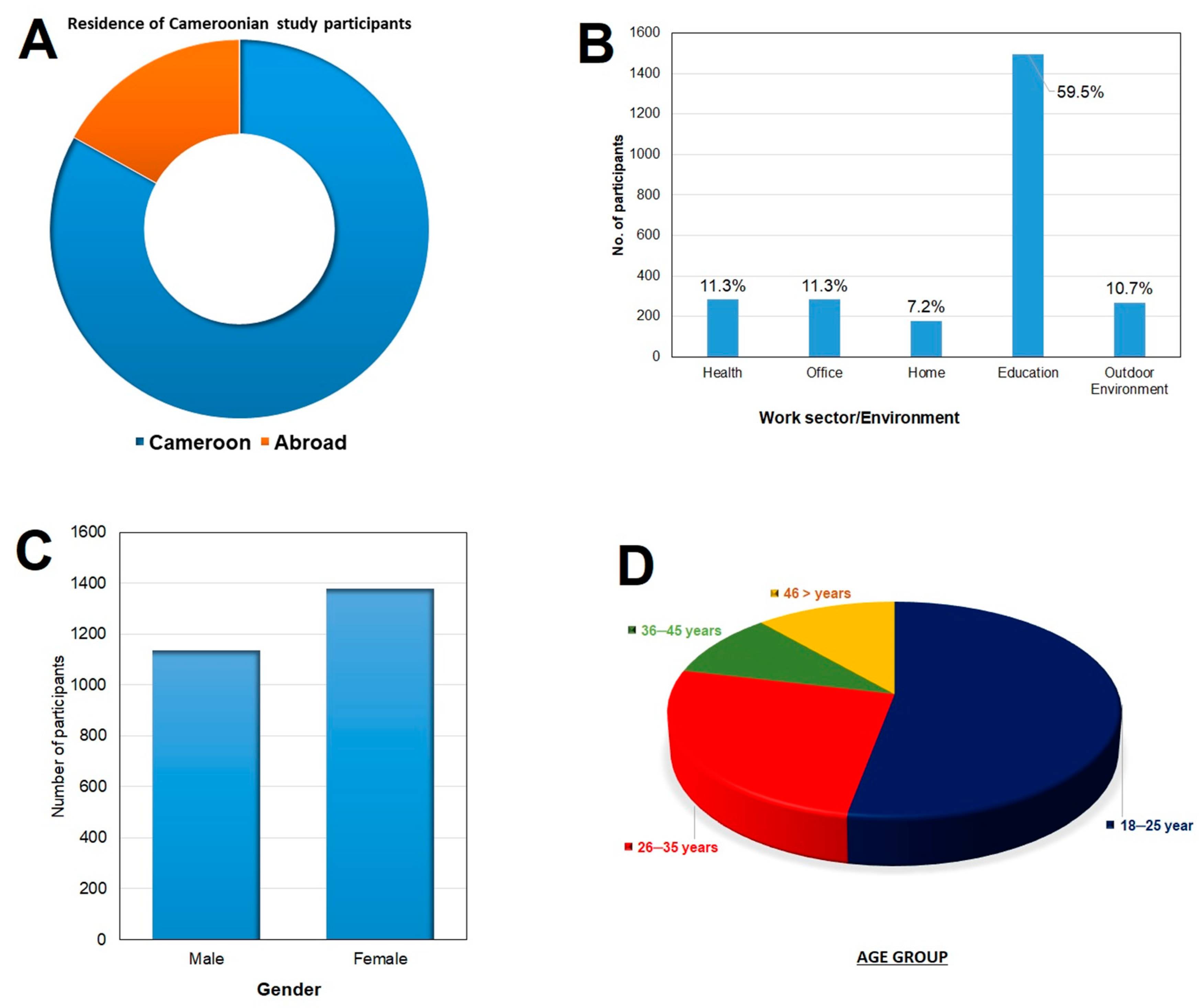
Vaccines Free Full Text Assessment Of Vaccine Hesitancy To A Covid 19 Vaccine In Cameroonian Adults And Its Global Implication Html
Covid 19 Vaccine Brand Hesitancy And Other Challenges To Vaccination In The Philippines Plos Global Public Health
Covid 19 Vaccine Brand Hesitancy And Other Challenges To Vaccination In The Philippines Plos Global Public Health
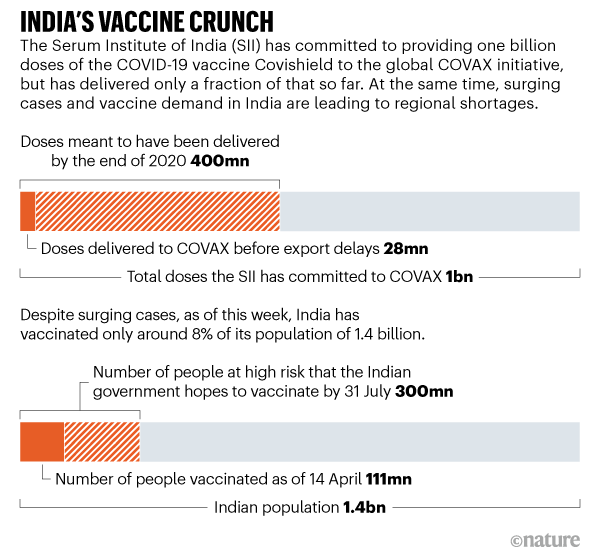
India S Covid Vaccine Woes By The Numbers
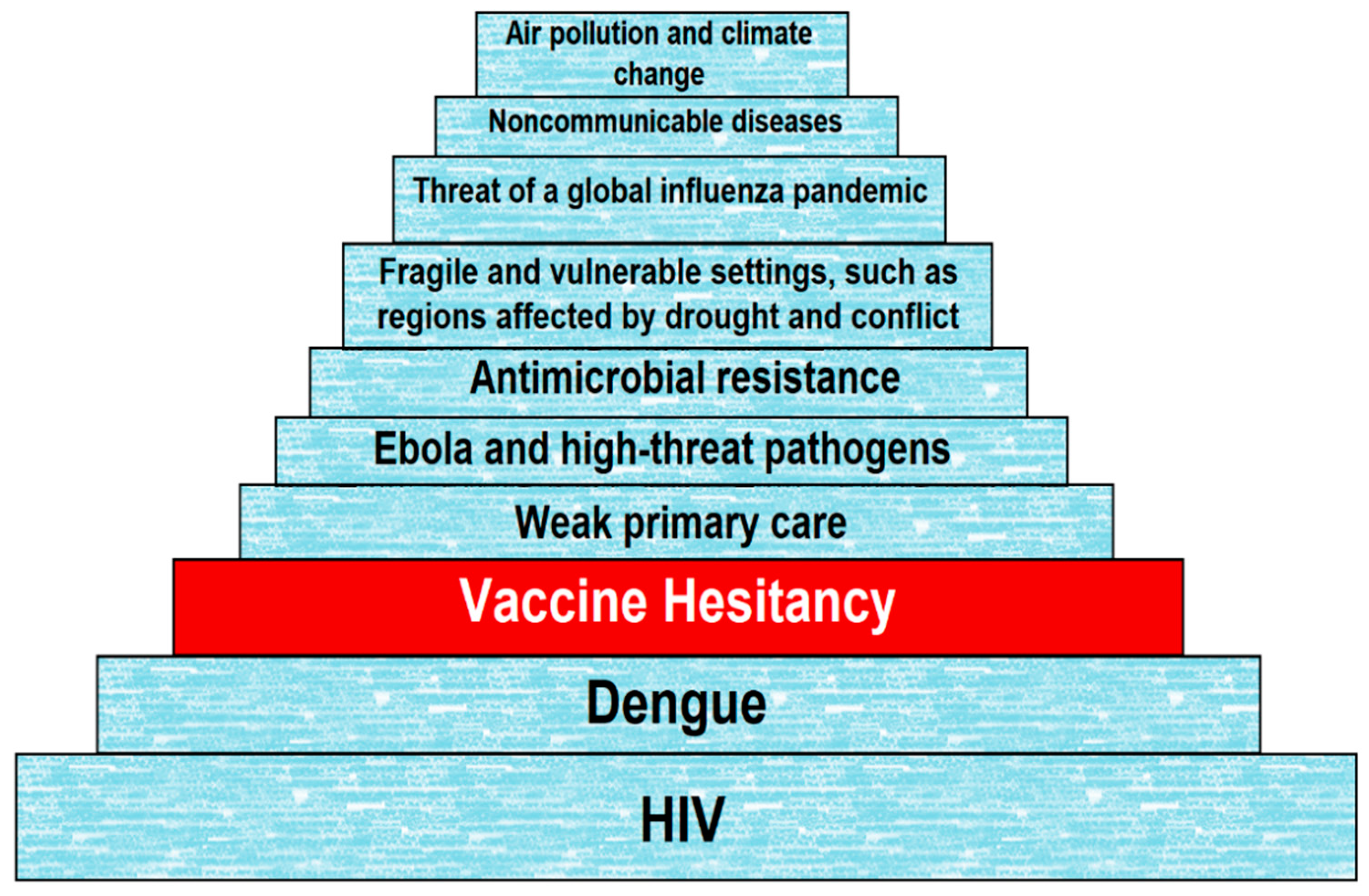
Vaccines Free Full Text Assessment Of Vaccine Hesitancy To A Covid 19 Vaccine In Cameroonian Adults And Its Global Implication Html

Types Of Vaccines Infographics Epidemiology Covid 19 Response Corps